Abstract
Introduction: Recent genetic discoveries involved in hematologic malignancies have resulted in breakthroughs in novel therapeutic candidate drugs. Nevertheless, drug discovery and development is a long, costly, and high-risk process in which 90% of drug candidates fail. Here, we analyzed phase 1 anti-leukemic drugs presented at ASH meetings between 2011 and 2015 as they progressed to later stage studies, publication and FDA approval.
Methods: We designed a customized hand-searching strategy to identify potentially relevant studies through the ASH publications website. We based our inclusion criteria on abstracts reporting clinical trials (CTs) that assessed anti-leukemia agents to treat leukemias, MDS, and MPS. We categorized the abstracts into main report, sub-analysis, or update. We searched the ClinicalTrials.gov database, PubMed, and FDA website to identify if CTs had transitioned to subsequent phase studies, were published as full-length journal manuscripts, and/or approved by the FDA. We calculated success rates in CTs from 2011 based on a 10-year timeline to approval.
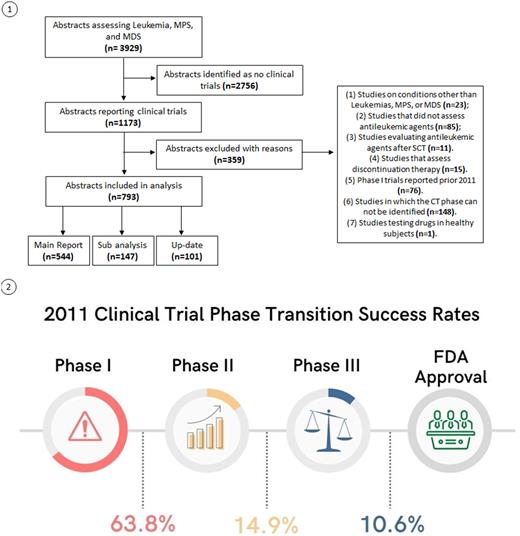
Results: We identified 3929 abstracts between 2011 and 2015 and determined that 1173 were CTs. After we applied our exclusion criteria, 793 studies remained, of which 545 were main CT reports, including 260 phase I, 232 phase II, 52 phase III, and 1 phase IV CTs. We identified 147 studies as sub-analysis and 101 as data updates (Figure 1).
Among the 260 phase I abstracts identified, 47 were identified from 2011; these were further explored. Nearly half of the studies involved AML (46.48%), followed by MDS (15.49%), CLL (14.08%), ALL (9.86%), CML (8.45%), and MPS (5.63%). Thirty-eight abstracts (80.85%) resulted in full-length manuscripts in journals such as Blood (36.84%), Clinical Cancer Research (21.05%), Haematologica (21.05%) and The New England Journal of Medicine (10.52%).
Most of the phase I CTs indicated that the drug was well tolerated (76.6%), effective (59.57%), and 8.5% of the abstracts conclusions described the drug as promising or encouraging. However, the transition to phase II success rate was 65.96%, with AML and CLL accounting for most (60% and 50%, respectively). Transition success to phase III was substantially lower (15.22%). The highest-performing diseases in this phase were CML and MPS (100% each), CLL (50%), MDS (25%) and AML (16.67%). Ultimately, we found that nearly one in ten (10.64%) of drugs in phase I CTs were approved by the FDA. CLL, CML, MPS (100% each) have the highest performing rates that led to regulatory approval, followed by AML (33.33%). (Figure 2). Of note, one CT evaluating a single agent (Glasdegib) was subsequently approved for AML in combination with another agent, and two drugs (Ponatinib and Ropeginterferon alfa-2b) transitioned from phase II to regulatory approval.
Conclusion: The clinical trial success rate in 2011 remains similar to that reported in the last few decades, with transition to phase II being the largest hurdle. Although acute leukemias were the most studied conditions, they presented the lowest performing rates, highlighting the challenges for drug development in this setting. We are continuing to evaluate investigational drugs over a 10-year period (2011-2020), and updated results will be presented at the meeting.
Disclosures
Cortes:Gilead: Consultancy; Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Sun Pharma: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Kartos: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.